



NOW COVERED
BY THE RAMQ ‡†6
Pheburane® is covered by most private and provincial/federal insurance plans
(British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec‡, Nova Scotia, New Brunswick, Newfoundland and Labrador, NIHB) (special authorization; criteria in addition to the indicated condition apply).2-12
Pheburane® (sodium phenylbutyrate) is indicated as adjunctive therapy in the chronic management of urea cycle disorders (UCD), involving deficiencies of carbamylphosphate synthetase, ornithine transcarbamylase or argininosuccinate synthetase. Pheburane® should be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, and protein-free calorie supplements).1
Pheburane® is indicated in patients with neonatal-onset presentation (complete enzyme deficiencies, presenting within the first 28 days of life). It is also indicated in patients with late-onset disease (partial enzyme deficiencies, presenting after the first month of life) who have a history of hyperammonemic encephalopathy.1
† Official mark of the Régie de l’assurance maladie du Québec
Click here for additional safety information and for a link to the Product Monograph discussing:
- Contraindications in patients with hypersensitivity to sodium phenylbutyrate or to any ingredient in the formulation, who are pregnant or breastfeeding.
- Relevant warnings and precautions in the management of acute hyperammonemia... routine monitoring during treatment.
- Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
Coated Granules1
Pheburane® consists of white to off-white tasteless coated granules and is available in a child-resistant high-density polyethylene (HDPE) bottle with a desiccant in the cap. 1
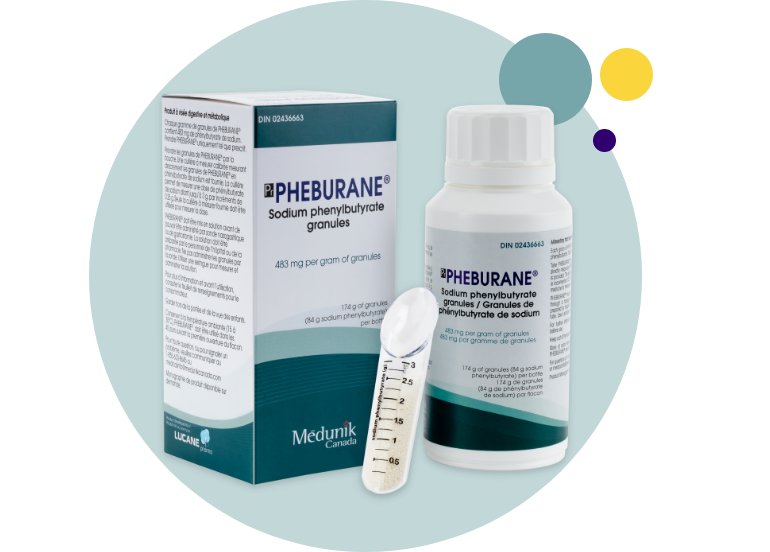
Dosage Forms and Packaging
Each bottle contains 174 g of granules, and each gram of granules contains 483 mg of sodium phenylbutyrate for a total of 84 g of sodium phenylbutyrate per bottle. 1
A calibrated measuring spoon is provided in the packaging.1
Dosing and Administration
Pheburane® should be administered orally. For patients unable to take the product orally, a solution of Pheburane® may be administered by nasogastric or gastrostomy tube (see Administration by nasogastric or gastrostomy tube in the Product Monograph). 1
Oral administration:
The total daily dose of Pheburane® should be divided into equal portions and taken with each meal or feeding (e.g., 4 to 6 times daily in young children). Granules can be swallowed with a drink or sprinkled on solid food; the mixture must be consumed immediately.
A calibrated measuring spoon is supplied. It measures Pheburane® granules directly in sodium phenylbutyrate up to 3 g in 0.25 g increments. Only the supplied spoon should be used. 1
Recommended Dose and Dosage Adjustment
Each gram of Pheburane® granules contains 483 mg of sodium phenylbutyrate. The usual total daily dose is:
- 450 to 600 mg/kg/day in children under 20 kg.
- 9.9 to 13.0 g/m²/day in patients ≥ 20 kg.
The safety and efficacy of doses > 20 g/day have not been established.
The measured amount using the calibrated spoon reflects sodium phenylbutyrate directly.
Table 3 – Doses for children under 20 kg
| Weight (kg) | Dosing interval | |
|---|---|---|
| Minimum dose (mg/day) | Maximum dose (mg/day) | |
| 3 | 1350 | 1800 |
| 4 | 1800 | 2400 |
| 5 | 2250 | 3000 |
| 7.5 | 3375 | 4500 |
| 10 | 4500 | 6000 |
| 15 | 6750 | 9000 |
| 20 | 9000 | 12000 |
Table 4 – Doses for patients 20 kg and over
| Body Surface Area (m²) | Dosing interval | |
|---|---|---|
| Minimum dose (g/day) | Maximum dose (g/day) | |
| 0.8 | 7.9 | 10.4 |
| 1.05 | 10.4 | 13.7 |
| 1.27 | 12.6 | 16.5 |
| 1.48 | 14.7 | 19.2 |
| 1.66 | 16.4 | 20.0* |
| 1.84 | 18.2 | 20.0* |
| 1.97 | 19.5 | 20.0* |
* The safety and efficacy of doses > 20 g/day have not been established.
For patients weighing less than 20 kg (44 lbs), the dose is based on weight:
please enter ONLY the patient’s weight below (select imperial or metric units), and then click "Calculate".
- Calculator will display the appropriate minimum and maximum daily dose of sodium phenylbutyrate.
For patients weighing equal to or more than 20 kg (44 lbs), the dose is based on Body Surface Area (BSA):
please enter the patient’s height and weight below (select imperial or metric units), and then click "Calculate".
- Calculator will determine the patient’s Body Surface Area (BSA) using the Du Bois method*. Other methods can be used for calculating BSA.
- Calculator will then display the appropriate minimum and maximum daily doses of sodium phenylbutyrate.
Please consult the Pheburane® Product Monograph for complete dosing information.
Covered by most private and provincial/federal insurance plans 1
(British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec‡, Nova Scotia, New Brunswick, Newfoundland and Labrador, NIHB) (special authorization; criteria in addition to the indicated condition apply).2-12
‡ In association with dietary protein restriction, for the treatment of patients suffering from a carbamoyl phosphate synthetase deficiency, an ornithine transcarbamylase deficiency or an argininosuccinate synthetase deficiency, whose plasma ammonium level is inadequate despite a sodium benzoate treatment at the optimal dose, unless there is an important intolerance or a contraindication to this drug. The maximum duration of each authorization is 12 months. When requesting continuation of treatment, the prescriber must provide evidence of a beneficial clinical effect.
Contact a Medunik Representative† Official mark of the Régie de l’assurance maladie du Québec